Association of Induction Immunosuppression Agents with Incidence of Cytomegalovirus Infection Following Kidney Transplantation
Medicine- Nephrology, University of Wisconsin, Madison, WI
Meeting: 2019 American Transplant Congress
Abstract number: B221
Session Information
Session Name: Poster Session B: Kidney Infections
Session Type: Poster Session
Date: Sunday, June 2, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose:
Cytomegalovirus (CMV) is the most common opportunistic infection following renal transplantation and can lead to significant morbidity. Inconsistency in adjusting for donor/recipient pre transplant CMV serostatus and in Alemtuzumab dosing probably contributed to conflicting results of studies on CMV infection rates with use of different induction agents. Here we report on first year post-transplant CMV infection incidence following induction with Thymoglobulin (ATG), Alemtuzumab in two different doses and Basiliximab as this may assist in decision making with regards to induction therapy. .
*Methods: This was a longitudinal single center study in Kidney transplant recipients between 2000 and 2006 (early era: EE), and 2011 to 2015 (late era: LE). The dose of Alemtuzumab used in the EE was 20 mg on day 0 and on day 1 post transplant, whereas a single 30 mg dose was used in the LE. Alemtuzumab usage for induction ceased in between the two eras. Subjects were followed up for one year post transplantation or until the occurrence of CMV infection, defined as the detection of viral replication by PCR.
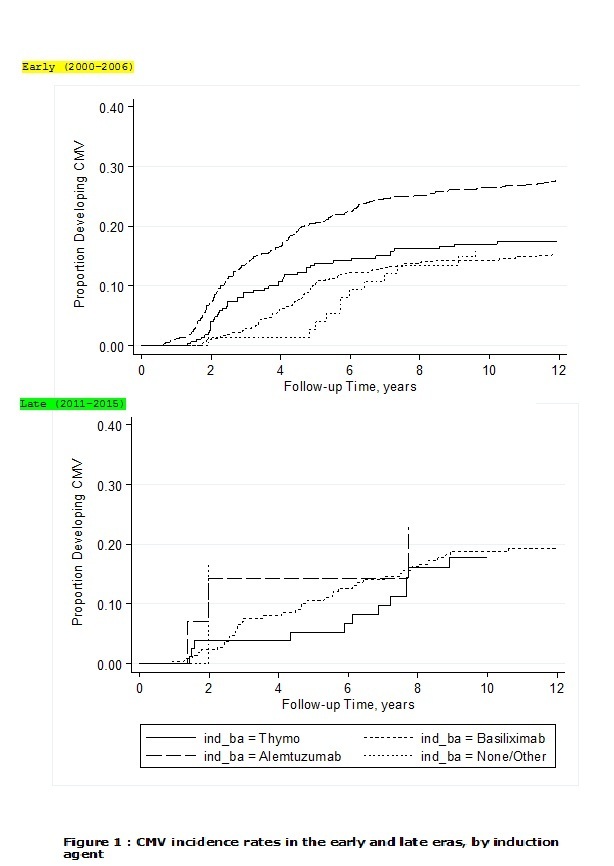
*Results: There were 2060 subjects who received one of the three induction agents, 1714 recipients in the EE and 346 in the LE. The Alemtuzumab group included more high CMV risk (donor positive/recipient negative) cases compared to ATG, without a significant difference from the Basiliximab group. Incidence rates of CMV infection in the EE were higher in the Alemtuzumab group compared to ATG and Basiliximab (34.9 versus 20, and 16.9 per 100 patient years respectively, P < 0.001). In the LE, no significant difference was found in CMV infection rates (27.4 ,19.4 and 22 per 100 patient years in the Alemtuzumab, ATG and Basiliximab groups respectively, both p=0.6 vs. Alemtuzumab) (Figure 1). Alemtuzumab was associated with a propensity-score adjusted hazard ratio (HR) of 1.72 (95% CI: 1.25, 2.37) compared to ATG in the EE (p=0.001), whereas the adjusted HR for Alemtuzumab compared to ATG in the LE was 1.07 (p=0.91).
*Conclusions:
In kidney transplant recipients, induction with Alemtuzumab was associated with a higher incidence of CMV infection compared to induction with ATG in the first post-transplant year when two 20 mg doses were used, with no difference seen when given as a single 30 mg dose, and no difference with either doses when compared to basiliximab. Further studies investigating the frequency of CMV infection following induction with different agents in high and intermediate CMV risk patients are warranted.
To cite this abstract in AMA style:
Medani SA, Astor BC, Djamali A, Mohamed MA. Association of Induction Immunosuppression Agents with Incidence of Cytomegalovirus Infection Following Kidney Transplantation [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/association-of-induction%e2%80%afimmunosuppression-agents-with-incidence-of-cytomegalovirus-infection%e2%80%affollowing-kidney-transplantation/. Accessed March 3, 2026.« Back to 2019 American Transplant Congress